
Haemophilius Type B Conjugate
Indications
Indicated for active immunization against Haemophilus Influenza Type B infection for all children from age 6 weeks to 5 years.
Dosage
0.5 ml injected intramuscularly into the anterior–lateral aspect of the thigh in infants or into the deltoid muscle of older children.
Recommended Immunization schedule:
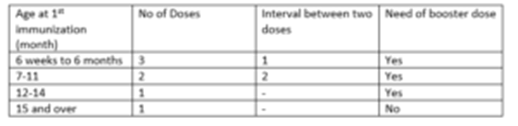
All vaccinated children should be given a single booster dose at 12 to18 months of age, but the interval after the previous dose should not be less than 2 months. Previously unvaccinated children 15 months to 5 years of age receive a single injection of Sii HibPro.
Interruption of the recommended schedules with a delay between doses does not interfere with the final immunity achieved nor does it necessitate starting the series over again regardless of the length of time elapsed between doses.
Note
Babies born preterm should be vaccinated according to their consecutive age, from birth,
Shelf Life
The vaccine should be injected promptly after reconstitution.
Contraindications
• Hypersensitivity to the vaccine.
• The vaccination must be provided to children with moderate or severe febrile illness as soon as they recover from the acute phase of the illness.
Precautions
• Since the possibility of anaphylactoid or other allergic-type reactions is possible following administration of the Hib Vaccine, 1: 1000 Adrenaline should be available for immediate treatment if such reactions occur. Therefore, the vaccine should be under medical supervision for at least half an hour after immunization.
• Use by pregnant women is not recommended.
Drug Interaction
• Individuals taking immunosuppressive therapy (e.g. Corticotrophin, corticosteroids, alkaline agents, antimetabolites, radiation therapy) may have a diminished antibody response to immunization with Sii Hib Pro.
• Hib vaccine can be administered in a safe and effective way at the same time as BCG, DTP, measles, polio vaccines (OPV or IPV), HBV, or yellow fever vaccines.
• The vaccine can be administered either simultaneously or at any time before or after a different inactivated or live vaccine but separately at different sites.
Adverse reactions
Localized reactions may occur within 24 hours of vaccination, when recipients may experience pain and tenderness at the injection site. These reactions are generally mild and transient and in most cases spontaneously resolve within 2-3 days.
Systemic
The general symptoms reported within the first 48 hours include fever, loss of appetite, restlessness, vomiting, diarrhea, and unusual crying.