
Human Papiloma virus
Therapeutic Indications
Females from 9 – 45 years of age for the prevention of cervical cancer by protecting against incident and persistent infections, cytological abnormalities including atypical squamous cells of undetermined significance (ASCUS) and cervical intraepithelial neoplasia (CIN), CIN 1 and precancerous lesions (CIN 2 and CIN 3) caused by human papillomavirus types 16 and 18.
Method of administration
It depends on the age of the subjects.
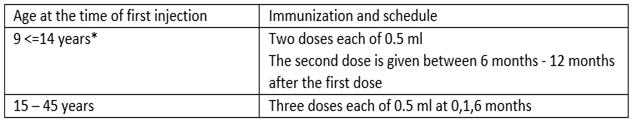
A third dose should always be administered if the second vaccine dose is received before the 5th month after the first dose.
If flexibility in the vaccination schedule is necessary, the second dose can be administered between 1 month and 2, 5 months after the first dose, and the third dose between 5 to 12 months after the first dose.
The need for the booster dose has not been established.
Pediatric Population
It is not recommended for use in girls below 9 years of age due to a lack of data on safety and immunogenicity in this age- group.
Method of administration
Intramuscular injection in the deltoid region.
Contraindications
• Hypersensitivity to active substances
• Pregnancy
• Lactation
Administration of vaccines should be postponed in patients suffering from an acute severe febrile illness. However, a minor infection such as a cold is not a contraindication for immunization.
Adverse Reactions
Very common: Pain at the injection site, Headache, rash, Myalgia, Nausea, vomiting
Common: Fever
Other: Arthralgia
Special warnings and precautions for use
• The vaccination of an individual woman should take into account her risk for previous HPV exposure and her likely benefit from vaccination.
• It will only protect against diseases caused by HPV types 16 and 18 and to some extent against diseases caused by certain other oncogenic-related HPV types. Therefore, appropriate precautions against STDs should continue to be used.
• It is for prophylactic use only and therefore, has no effect on active HPV infection or established clinical disease.